Featured Publication in Focus: Regulation of senescence-associated secretory phenotypes in osteoarthritis by cytosolic UDP-GlcNAc retention and O-GlcNAcylation
Mar 19 , 2025
Authors:
Donghyun Kang, Jeeyeon Lee, Geunho Yook, Sehan Jeong, Jungkwon Shin, Mi-Sung Kim, Yi-Jun Kim, Hyeryeon Jung, Jinsung Ahn, Tae Woo Kim, Moon Jong Chang, Chong Bum Chang, Seung-Baik Kang, Won Ho Yang, Yong-ho Lee, Jin Won Cho, Eugene C. Yi, Chanhee Kang, Jin-Hong Kim
Department of Biological Sciences, College of Natural Sciences, Seoul National University, Seoul 08826, South Korea.
nature communications. nature
----------------------
Product referenced:
Catalogue # 1042003
Aggrecan Antibody to C-terminal Neoepitope NITEGE Mouse Monoclonal Antibody
----------------------
ABSTRACT
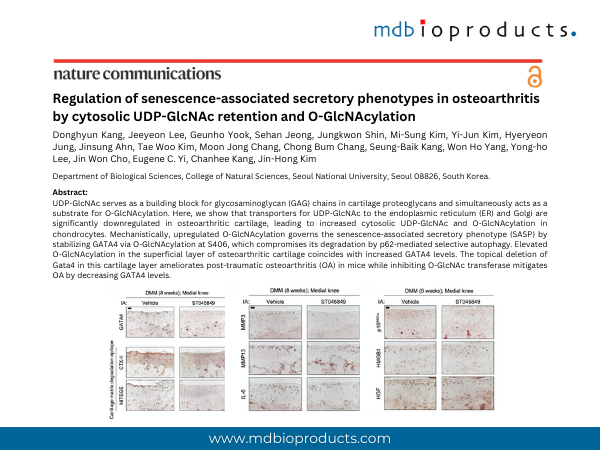
UDP-GlcNAc serves as a building block for glycosaminoglycan (GAG) chains in cartilage proteoglycans and simultaneously acts as a substrate for O-GlcNAcylation. Here, we show that transporters for UDP-GlcNAc to the endoplasmic reticulum (ER) and Golgi are significantly downregulated in osteoarthritic cartilage, leading to increased cytosolic UDP-GlcNAc and O-GlcNAcylation in chondrocytes. Mechanistically, upregulated O-GlcNAcylation governs the senescence-associated secretory phenotype (SASP) by stabilizing GATA4 via O-GlcNAcylation at S406, which compromises its degradation by p62-mediated selective autophagy. Elevated O-GlcNAcylation in the superficial layer of osteoarthritic cartilage coincides with increased GATA4 levels. The topical deletion of Gata4 in this cartilage layer ameliorates post-traumatic osteoarthritis (OA) in mice while inhibiting O-GlcNAc transferase mitigates OA by decreasing GATA4 levels. Excessive glucosamine-induced O-GlcNAcylation stabilizes GATA4 in chondrocytes and exacerbates post-traumatic OA in mice. Our findings elucidate the role of UDP- GlcNAc compartmentalization in regulating secretory pathways associated with chronic joint inflammation, providing a senostatic strategy for the treat- ment of OA.
To continue reading and to download the publication:
