Featured Publication in Focus: Repeated immunization with ATRA-containing liposomal adjuvant transdifferentiates Th17 cells to a Tr1-like phenotype
Jul 08 , 2024
Authors:
Katharina Wørzner, Julie Zimmermann, Regitze Buhl, Anna Desoi, Dennis Christensen, Jes Dietrich, Nina Dieu Nhien Tran Nguyen, Thomas Lindenstrøm, Joshua S. Woodworth, Reham Sabah Alhakeem, Steven Yu, Niels Ødum, Rasmus Mortensen, Judith F. Ashouri, Gabriel K. Pedersen.
Center for Vaccine Research, Statens Serum Institut, Copenhagen, Denmark
ScienceDirect. Journal of Autoimmunity
----------------------
Product referenced:
Catalogue # 3038001
Myelin Oligodendrocyte Glycoprotein (MOG 35-55), 25 mg
----------------------
ABSTRACT
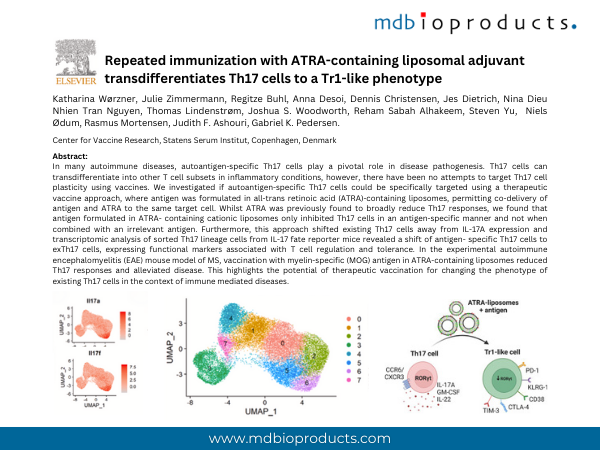
In many autoimmune diseases, autoantigen-specific Th17 cells play a pivotal role in disease pathogenesis. Th17 cells can transdifferentiate into other T cell subsets in inflammatory conditions, however, there have been no attempts to target Th17 cell plasticity using vaccines. We investigated if autoantigen-specific Th17 cells could be specifically targeted using a therapeutic vaccine approach, where antigen was formulated in all-trans retinoic acid (ATRA)-containing liposomes, permitting co-delivery of antigen and ATRA to the same target cell. Whilst ATRA was previously found to broadly reduce Th17 responses, we found that antigen formulated in ATRA-containing cationic liposomes only inhibited Th17 cells in an antigen-specific manner and not when combined with an irrelevant antigen. Furthermore, this approach shifted existing Th17 cells away from IL-17A expression and transcriptomic analysis of sorted Th17 lineage cells from IL-17 fate reporter mice revealed a shift of antigen-specific Th17 cells to exTh17 cells, expressing functional markers associated with T cell regulation and tolerance. In the experimental autoimmune encephalomyelitis (EAE) mouse model of MS, vaccination with myelin-specific (MOG) antigen in ATRA-containing liposomes reduced Th17 responses and alleviated disease. This highlights the potential of therapeutic vaccination for changing the phenotype of existing Th17 cells in the context of immune mediated diseases.
To continue reading and to download the publication: https://doi.org/10.1016/j.jaut.2024.103174
