Featured Publication in Focus: Selective disruption of Traf1/cIAP2 interaction attenuates inflammatory responses and limits sepsis and rheumatoid arthritis
Jan 28 , 2025
Authors:
Yitian Tang, Fatemah Aleithan, Sahib Singh Madahar, Ali Mirzaesmaeili, Sunpreet Saran, Jialing Tang, Safoura Zangiabadi, Robert Inman, Gary Sweeny and Ali A. Abdul-Sater
School of Kinesiology and Health Science, Muscle Health Research Centre, York University, Toronto, Ontario, Canada
Cold Spring Harbor Laboratory. bioRxiv
----------------------
Product referenced:
Catalogue # CIA-MAB-2C
ArthritoMab™ Antibody Cocktail for C57BL/6, TG, 50 mg
----------------------
ABSTRACT
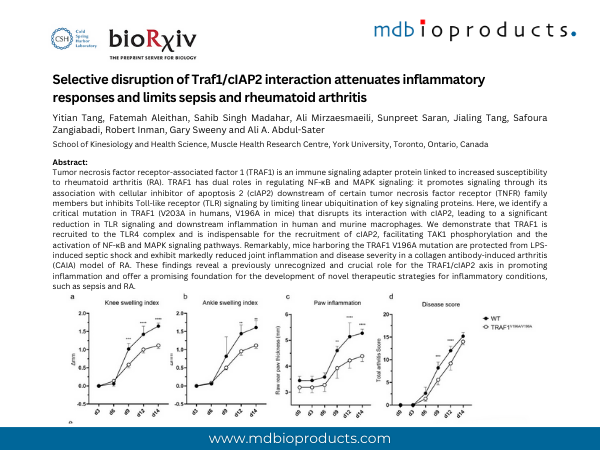
Tumor necrosis factor receptor-associated factor 1 (TRAF1) is an immune signaling adapter protein linked to increased susceptibility to rheumatoid arthritis (RA). TRAF1 has dual roles in regulating NF-κB and MAPK signaling: it promotes signaling through its association with cellular inhibitor of apoptosis 2 (cIAP2) downstream of certain tumor necrosis factor receptor (TNFR) family members but inhibits Toll-like receptor (TLR) signaling by limiting linear ubiquitination of key signaling proteins. Here, we identify a critical mutation in TRAF1 (V203A in humans, V196A in mice) that disrupts its interaction with cIAP2, leading to a significant reduction in TLR signaling and downstream inflammation in human and murine macrophages. We demonstrate that TRAF1 is recruited to the TLR4 complex and is indispensable for the recruitment of cIAP2, facilitating TAK1 phosphorylation and the activation of NF-κB and MAPK signaling pathways. Remarkably, mice harboring the TRAF1 V196A mutation are protected from LPS-induced septic shock and exhibit markedly reduced joint inflammation and disease severity in a collagen antibody-induced arthritis (CAIA) model of RA. These findings reveal a previously unrecognized and crucial role for the TRAF1/cIAP2 axis in promoting inflammation and offer a promising foundation for the development of novel therapeutic strategies for inflammatory conditions, such as sepsis and RA.
To continue reading and to download the publication:
