Featured Publication in Focus: SR-BI regulates the synergistic mast cell response by modulating the plasma membrane-associated cholesterol pool
Jun 17 , 2024
Authors:
Sandro Capellmann, Marlies Kauffmann, Michel Arock and Michael Huber
Institute of Biochemistry and Molecular Immunology, Medical Faculty, RWTH Aachen University, Aachen, Germany
Wiley. European Journal of Immunology
----------------------
Product referenced:
Catalogue # 101001F
T1/ST2 (IL-33R) Mouse, Monoclonal Antibody, FITC, 0.5 mL
----------------------
ABSTRACT
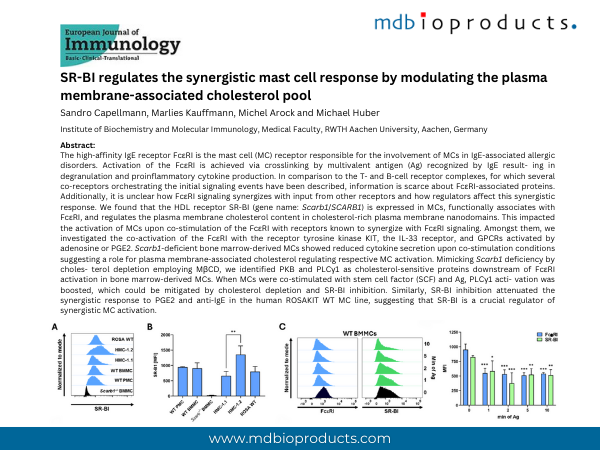
The high-affinity IgE receptor FcεRI is the mast cell (MC) receptor responsible for the involvement of MCs in IgE-associated allergic disorders. Activation of the FcεRI is achieved via crosslinking by multivalent antigen (Ag) recognized by IgE result- ing in degranulation and proinflammatory cytokine production. In comparison to the T- and B-cell receptor complexes, for which several co-receptors orchestrating the initial signaling events have been described, information is scarce about FcεRI-associated proteins. Additionally, it is unclear how FcεRI signaling synergizes with input from other receptors and how regulators affect this synergistic response. We found that the HDL receptor SR-BI (gene name: Scarb1/SCARB1) is expressed in MCs, functionally associates with FcεRI, and regulates the plasma membrane cholesterol content in cholesterol-rich plasma membrane nanodomains. This impacted the activation of MCs upon co-stimulation of the FcεRI with receptors known to synergize with FcεRI signaling. Amongst them, we investigated the co-activation of the FcεRI with the receptor tyrosine kinase KIT, the IL-33 receptor, and GPCRs activated by adenosine or PGE2. Scarb1-deficient bone marrow-derived MCs showed reduced cytokine secretion upon co-stimulation conditions suggesting a role for plasma membrane-associated cholesterol regulating respective MC activation. Mimicking Scarb1 deficiency by choles- terol depletion employing MβCD, we identified PKB and PLCγ1 as cholesterol-sensitive proteins downstream of FcεRI activation in bone marrow-derived MCs. When MCs were co-stimulated with stem cell factor (SCF) and Ag, PLCγ1 acti- vation was boosted, which could be mitigated by cholesterol depletion and SR-BI inhibition. Similarly, SR-BI inhibition attenuated the synergistic response to PGE2 and anti-IgE in the human ROSAKIT WT MC line, suggesting that SR-BI is a crucial regulator of synergistic MC activation.
To continue reading and to download the publication:
